Descoberta nova forma de radioatividade
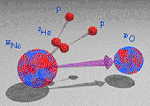 Segundo a Physics News Update (notícias de física enviadas por e-mail), um experimento conduzido no Istituto Nazionale di Fisica Nucleare, Itália, descobriu um novo decaimento radioativo, por emissão de dois prótons ligados, que podemos entender como um núcleo de He-2 (hélio-2). Este núcleo é altamente instável e também decai rapidamente. No experimento conduzido pelos pesquisadores, eles observaram os fragmentos da colisão de Ne-18 com chumbo. Neste processo, o Ne-18 se torna bastante excitado, decaindo em O-16 e He-2. Este tipo de radiação era previsto teoricamente há aproximadamente 50 anos. Os resultados deste trabalho foram publicados na Phys. Rev. Lett. 100, 192503 (2008). Na continuação eu transcrevo o e-mail com a notícia (obrigado ao Manfredo que me enviou cópia do e-mail).
Segundo a Physics News Update (notícias de física enviadas por e-mail), um experimento conduzido no Istituto Nazionale di Fisica Nucleare, Itália, descobriu um novo decaimento radioativo, por emissão de dois prótons ligados, que podemos entender como um núcleo de He-2 (hélio-2). Este núcleo é altamente instável e também decai rapidamente. No experimento conduzido pelos pesquisadores, eles observaram os fragmentos da colisão de Ne-18 com chumbo. Neste processo, o Ne-18 se torna bastante excitado, decaindo em O-16 e He-2. Este tipo de radiação era previsto teoricamente há aproximadamente 50 anos. Os resultados deste trabalho foram publicados na Phys. Rev. Lett. 100, 192503 (2008). Na continuação eu transcrevo o e-mail com a notícia (obrigado ao Manfredo que me enviou cópia do e-mail).
"NEW FORM OF ARTIFICIAL RADIOACTIVITY
The basic structure of matter has been known for almost a century, and yet scientists keep learning new things by persistently poking and ripping apart atoms. An atom consists of a relatively heavy part at the core, the nucleus, and a lighter part, a fleet of electrons, orbiting the nucleus. The electron part determines all the important chemical, electrical, and optical properties of the atom, but the nucleus is important too. It contains most of the atom’s mass and energy, and the reactions among nuclei are responsible for powering the sun.
Nature often plays tricks. Usually hydrogen atoms have a nucleus with a lone proton, but sometimes that nucleus can possess a neutron in addition. This version, or isotope, of hydrogen is called H-2 since it has two nuclear units. Still another version of hydrogen, H-3, has a nucleus consisting of one proton and two neutrons. Similarly, the main form of helium, He-4, has four nuclear particles, but can also get by with only three: the He-3 isotope consists of two protons and one neutron. All the other elements also have numerous isotopes, some of which are stable, which means they can persist for millions of years, and some are unstable, which means that they break apart after a certain typical period called a half-life.
Radioactivity is the process by which unstable nuclei transform into more stable nuclei. “Radio� refers not to the kind of radio waves we get from a station but to the castoffs---either in the form of particles or electromagnetic waves---radiated by the parent nucleus. Historically the main forms of radioactivity were identified as alpha, beta, and gamma rays (these being the first three letters of the Greek alphabet). An alpha ray or alpha particle is none other than a He-4 nucleus. Beta rays are now known to be electrons. And gamma rays are really just high-energy waves, even more potent than x rays. The new kind of radioactivity, discovered in an experiment conducted recently at the Istituto Nazionale di Fisica Nucleare, a nuclear laboratory in Italy, consists of nuclear fragments made of two protons. You can think of this as a new isotope of helium. He-2, as it would be called, is highly unstable and very quickly flies apart. Making the unexpected new nuclear species took some ingenuity. First a beam of neon-20 ions was crashed into a foil of beryllium. In this collision some of the neon nuclei suffered a slight robbery: losing two protons they ended up as neon18 nuclei. Next, these same flying nuclei encountered a foil of lead. This second collision had the effect of exciting the Ne-18 nucleus into a highly unstable condition. The remedy for this instability was for the Ne-18 nucleus to slough off a fragment. There are several ways of doing this. Among the decay options, the Italian physicists found, was a rare, never-before-demonstrated process in which the Ne-18 nucleus turned itself into an oxygen-16 nucleus, plus that He-2 fragment. According to one of the researchers, Giovanni Raciti at the LNS-INFN lab (raciti@lns.infn.it), the two-proton decay mode was predicted about 50 years ago. A few experiments conducted before this showed ambiguous evidence: two protons emerged from the decay but one couldn’t tell that the protons had not been thrown out one at a time or both at the same time randomly from the whole Ne-18 or from a single lump. The new experiment definitely shows that the two protons come out together from the breakup of a He-2 cluster (see figure at http://www.aip.org/png/2008/302.htm). The new form of helium isn’t good for anything practical since it doesn’t survive even for a billionth of a second. Raciti believes, however, that the observation of this slender isotope of helium will us understand how are built very unstable nuclei with a number of protons exceeding the one of neutrons and, conversely, how heavy nuclei---the cores of the heavier atoms here on earth---are built up in the interiors of stars. (Physical Review Letters, 16 May 2008)"




No comments